Main Menu
About Ionode
Originally established in 1972 by well respected inventor and scientist John Petty, Ionode's reputation as a family business supporting a community of hard working individuals has grown stronger and stronger at each developmental milestone. Under new management since 2009, Ionode staff remain as long-term contributors to the success and reputation of the business and always go well beyond the norm to meet and exceed your expectations of quality and reliability in our products.
Developing and utilizing the latest innovations in electrode manufacturing technology, Ionode serves its global customers from a purpose built manufacturing facility located in Brisbane, Australia and is the largest manufacturer of electrochemical sensors in the southern hemisphere. Specialising in pH, ORP, ion selective and custom designed electrodes for over 50 years, Ionode has developed many advanced manufacturing techniques to stay at the fore-front of sensor reliability and the value for money equation. Underpinning this outstanding reliability is an automated glassblowing process which produces a variety of different pH membrane shapes to exacting standards and uses a uniform mass of pH glass to ensure a consistent, reliable result from all Ionode products. Driven by customer needs, R&D, constant innovation and outstanding QC procedures, Ionode products are relied upon in the most demanding applications imaginable and are the preferred choice in dozens of industries.
Trusted Worldwide
Ionode products are relied upon by the Australian & New Zealand Wine Industry, MLA (Meat & Livestock Association of Australia), Master Winemakers of Australia, global pool controller manufacturers, Universities and educational institutions, allied health, bio-medical, pharmaceuticals, mining, water operations and dozens more. Contact Ionode for more information.

Inline Sensors for Pool & Water Treatment Combined Sensors/Screw-in/Integrated
Whether in swimming pools, process control or other inline applications, reliability is everything and Ionode’s reputation for robust and reliable sensors has been well documented since 1972. Ionode offers multi-parameter sensors with up to four parameters in one sensor, direct replacement models for other brands and a custom sensor service to build to your own specifications ... all with reliability beyond the norms of other manufacturers.


Troubleshooting
Electrical interference problems are complex and cannot be easily presented in this forum. Noise, pH or ORP offset voltage, optical isolation and solution earth, differential amplifiers, single ended amplifiers, amplifier input impedance > 1x1012 Ohms and much more make this a job for experts ... discuss your needs with the Ionode Tech Team.
If you’re having technical difficulties with issues such as electrical interference, refer to this informative discussion extracted from a previous case...
.... the pH side of the probe develops a stable voltage between its output and the solution, which is dependent on the pH of the solution. The purpose of the Ag/AgCl reference then is to develop a stable voltage with respect to the solution, so you can complete the circuit and get the pH value. You can’t just use say a piece of metal as the reference because they develop a redox potential and the voltage isn’t stable. The way an ORP probe works is just that it measures the redox potential by having a piece of metal (typically platinum) in the solution, and it’s referenced to the Ag/AgCl reference in the probe.
Often in pools and industrial control applications a noise voltage develops between the solution and electrical earth. We assume that this happens because of poorly constructed pools, faulty pumps, or even static build up on the PVC pipes; however it's not something we or the controller manufacturer typically has control over and so we have to work around it.
The circuit diagrams should help explain the problem. The typical pH meter and electrode is shown in the 1st diagram. They use a single-ended amplifier for the pH signal. The pH electrode's reference is tied to the ground of the circuit, and so the voltage between them is assumed to be 0mV. When the ground of the pH circuit is otherwise untied, there is no problem, because it simply floats to the potential of the solution and there is no noise voltage applied across R_REFERENCE. For example, this is what happens when using a battery-powered handheld meter.
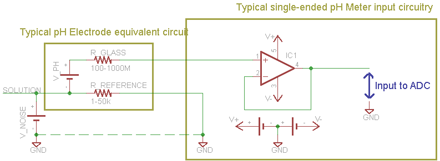
However, typical pool/control installations have a fixed power source which ties the ground potential to the same ground as the noise source, or another fixed potential with respect to the noise source. This in turn forces a noise voltage across R_REFERENCE which has a direct effect on the pH reading. Often the noise voltage is high enough to exceed the common mode input limits of the amplifier as well, causing it to saturate and even be damaged in some situations. In addition the extra current flowing through the electrode's reference polarises the reference giving another error source.
One solution to the problem is shown in the 2nd diagram. A differential instrumentation amplifier is used instead of the single-ended amplifier. The solution noise voltage is reduced to near 0 by the low resistance solution earth connector directly connected to the circuit ground; and the amplified voltage is now correctly the voltage between the pH and reference instead of the voltage between the pH and ground. Instrumentation amplifiers also typically have much better common mode rejection than traditional operational amplifier configurations.
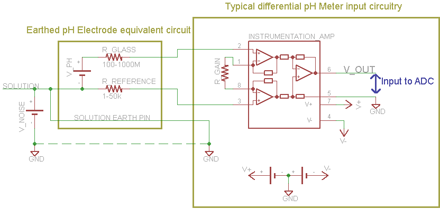
Recommended is the TI INA116 instrumentation amplifier and other manufacturers also have amplifiers which are suitable. There are likely other techniques that solve the problem as well - for example one could design their own instrumentation amplifier; or use optical isolation. The key specifications for pH amplifiers are that the input impedance is much higher (typically 1000x) than the electrode resistance, so as a rule the input impedance should be greater than 1x1012 ohms (at 25C); and for the same reason the input bias current must be very low, as a rule less than 5pA (at 25C). Be aware that the resistance of a pH electrode doubles with every 6~10C drop in temperature and so lower temperatures require better performance from the amplifier.

