Main Menu
Ion Selective Electrode Theory
Download this information for future reference
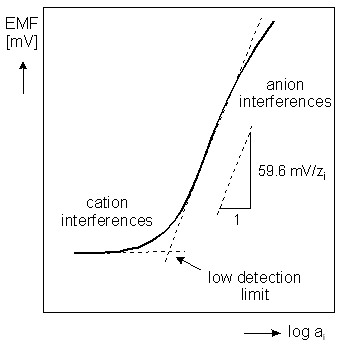
An Ion selective electrode is a sensor which converts the activity of a specific ion (dissolved in a solution) into a voltage (potential), which can be measured by a mV or Ion meter. The voltage is theoretically dependent on the logarithm of the ion activity, as described by the Nernst Equation.
E = E0 + (2.303*RT/nF)log(A)
The sensing part of the electrode is usually made from an ion specific membrane, coupled together with a reference electrode (either separate or as a combination).
There are five main types of ion selective membranes which are detailed hereunder.
One
Glass membrane electrodes are formed from special ionically conducting glass. By altering the composition and constituents of the glass, changes can be made to selectivity, chemical resistance, response time, and impedance. The most common glass membrane ion selective electrode is the pH electrode. Another common one is the sodium selective electrode.
Two
Crystalline or solid state membrane electrodes are made from relatively insoluble ionically conducting inorganic salts. These are available in homogeneous and heterogeneous forms. They have good selectivity since only ions which can introduce themselves into the crystal lattice can interfere. Examples include the Fluoride electrode which uses a doped LaF3 crystal, and the Chloride electrode which uses silver chloride powder.
Three
Polymer Membrane Electrodes are based on special organic polymer membranes which contain various ion-exchange ionophores incorporated into an inert matrix. These are used in electrodes to measure ions such as Potassium, Calcium, and Nitrate.
Four
Gas sensing electrodes have gas permeable membranes and an internal solution. Gas molecules diffuse across the membrane and react with the solution. This pH change is then detected by the pH electrode. Common gas sensing electrodes include ammonia and carbon dioxide.
Five
Enzyme electrodes are based on the reaction of an enzyme reacting with a specific substrate, and the resultant product of this reaction (usually H+ or OH-) is detected by an electrode such as a pH electrode. These reactions occur within a special membrane. An example is the Glucose electrode.
The Reference Electrode
An important part of the measurement is the use of a stable reference electrode. Many ISE's incorporate their own reference electrode; these are usually either a single junction refillable type Ag/AgCl type, or the double junction type, which is used for ISE's such as chloride, bromide etc. These types of reference electrodes allow the user to select an appropriate electrolyte for the particular application. For instance Potassium Nitrate is commonly used as a filling solution for ISE's such as Chloride, Bromide, Iodide, Cyanide, Silver and Sulphide.Whenever choosing a filling solution, it is also important to select one that is close to being equitransferant to minimise junction potential errors. The E0 factor in the Nernst equation is the sum of all the liquid junction potentials present in the system, and any variation in this during analysis can introduce major sources of potentail drift and error in measurements.
Use of ISA/TISAB
For accurate and reproducible measurements, adjustment of the ionic strength is often required. The reason is to raise the the ionic strength of all standards and samples to a uniformly high level so that the activity coefficient is the same for the ions in both solutions. This enables the concentration of the samples to be more accurately determined. ISA's can alo be used to adjust the pH of samples. TISAB (Total Ionic Strength Adjuster Buffer), used for Fluoride Ion analysis is a common example of this. This solution adjusts the ionic strength, adjusts the pH and can also minimise interferences.
Sample Pre-Treatment
It is important to note that Ion selective electrodes measure the activity of the ionic analytes in solution. If the ion to be measured is complexed or bound, sample pre-treatment may be required before analysis. This can involve preliminary steps such as drying, washing and grinding, as well as extraction or dry ashing. This is particularly the case with samples such as plant material and soils. In the event of interferences and oxidation effects, adding an appropriate reagent to both samples and standards can be beneficial.
ISE Analytical Techniques
 Direct Calibration
Direct Calibration
Simple procedure used for measuring a large number ofsamples. This can also easily be automated with sample changers for unattended analysis. Calibration is performed using a series of standards, preferably bracketing the expected sample range. ISA is added to both samples and standards in the same ratios, and the concentration of the samples is determined by comparison to the standards.
Standard Addition
The electrode is immersed in the sample solution and an aliquot of a standard solution containing the measured species is added to the sample. From the change in potential before and after the addition, the original sample concentration is determined.
Analate Addition
Often used to measure viscous samples, small or very concentrated samples. It can be used to overcome the effects of complex sample matrics, but is not suitable for dilute samples.Total concentration is measured even in the presence of complexing agents. The electrode is immersed in a standard solution containing the ion to be measured, and an aliquot of the sample is added to the standard. The original sample concentration is determined from the change in potential before and after the addition.
Analate Subtraction
Used for the measurement of ions for which no ISE exists. The electrode is immered in a reagent solution that contains an ion that the electrode senses, and which reacts with the sample. It is not suited for dilute samples. An example is using a Lead ISE to measure Sulphate.
Titration Indicators
Ion selective electrodes are very useful as end point detectors in titrations because they are unaffected by colour or turbidity. A common example is use of a chloride ISE (or a silver billet electrode) for determining the salt content of dairy products by silver nitrate titration. Titrations are much more precise (approx. 10x) than direct ISE calibration, but they are more time consuming and they have a narrower dynamic range. ISE's can also be used in titrations to determine ions for which there is no current ISE. For example, Lanthanum ions can be determined in this way using a Fluoride ISE as indicator.
Why Use Ion Selective Electrodes?
The main reasons Ion selective electrodes are popular:-
The initial set up is inexpensive (typically one only needs a pH/mV meter or Ion meter, the electrodes, a stirring stand, and some basic chemicals)
The measurements are unaffected by colour or turbidity in the sample
The sample pre-treatment is usually simple
The measurements can be done in “real time”, and can be easily automated
Wide dynamic range-usually several decades
Where Are They Used?
Ion selective electrodes or ISE’s are commonly used in environmental, food and agriculture, power plants, research and in clinical applications.
Examples include: Fluoride in drinking water, Calcium in beer, Nitrates in plants, Chloride in canned food products, and Calcium in dental studies.
Limitations
- selectivity/interferences
- accuracy, typically +/- 2-5% relative
- calibrating solutions and samples need to have the ionic strength adjusted
- lifetime and maintenance requirements of polymer membrane electrodes
- sample pre treatment is sometimes necessary for non-ideal samples
Care and Maintenance
Care must be taken to avoid damaging the membrane surface. Follow the instructions supplied with the electrodes to assure maximum life. After extensive use, the membranes may become coated with contaminants or become scratched. The solid state types such as Chloride and Fluoride can usually be easily repaired with fine emery and then washing with distilled water to remove debris. You should never touch the membranes of ISE's such as Calcium and Potassium, because it is very easy to damage the surface. These should instead be rinsed in standard solution and the deposits removed with a fine jet of water.
