Main Menu
ORP Theory
Download this information for future reference
ORP stands for oxidation reduction potential. Ideal ORP obeys the Nernst equation:
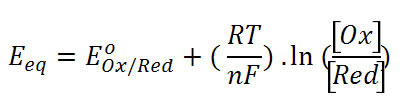
Where [Ox] is the active concentration of an oxidized species such as ferric ion Fe3+ and [Re] is the active concentration of the reduced form of that species namely ferrous ion Fe2+. The pair provides a redox couple.
The following is required for a stable ORP reading:
1) Significant concentrations of both species of the couple
2) Both species must be capable of readily transferring electrons to or from each other (reversible redox couple) and readily accepting or removing electrons from an inert metal surface.
Ferric/ferrous, iodine/iodide and quinone/hydroquinone are examples of reversible redox couples and are used for ORP standards.
Generally, most practical ORP measurements are made on samples which do not meet the above criteria. This results in poor reproducibility, drift, stirring rate dependence and non-Nernstian behaviour. Nevertheless, ORP is useful for measuring changes in a system rather than absolute values (e.g. process control to an ORP set-point and titrations).
Like pH, ORP represents an intensity factor. It does not characterize the capacity of the system for oxidation or reduction, in the same way pH does not characterize the buffering capacity.
Output
ORP values are determined by measuring the potential difference between an inert sensing half-cell and a reference half-cell as in pH. The sensing half-cell acts as a platform for electron transfer to or from the sample. The sensor does not undergo oxidation or reduction. It is typically platinum. Gold and Silver sensors can also be used. The former is substantially inert, whereas the latter responds to silver and halide ions, and can be used for silver titrations. The standard hydrogen electrode (SHE) is the reference from which all formal redox potentials are determined and has been assigned an arbitrary half cell potential of 0.0 mV. However, it is fragile and impractical for routine laboratory use. Therefore, Ag/AgCl and saturated calomel (SCE) reference electrodes are used. Earlier literature often used SCE. The use of SCE is now rare because it contains mercury. The voltages of the different reference electrodes can be interrelated (see Table 6). The IJ64 uses an Ag/AgC/saturated KCl reference electrode.
Table 6:Potential Relationships (mV) of Several Reference Electrodes at 25ºC
|
SHE |
SCE Saturated KCl |
Ag/AgCl/ 1M KCl |
Ag/AgCl/ 4M KCl |
Ag/AgCl Saturated KCl |
|
0 |
+245 |
+236 |
+200 |
+199 |
For example, a reading of 100mV using an Ag/AgCl/Saturated KCl reference could be referred to a SHE reference by adding 199mV.
Although the resistance of an ORP electrode is very low compared to a pH electrode, it is preferable to use a high impedance meter in order to avoid polarising the Ag/AgCl/sat KCl half cell.
Reference:
1) “2580 Oxidation Reduction Potential” Standard Methods for the Examination of Water and Wastewater, 19th edition, published by American Public Health Ass., American Water Works Ass., and Water Pollution Control Fed., 1995, 2-73.
Calibration Standards
Light's solution and ZoBell's solution are NBS standards which are stable for several months if stored in a dark plastic bottle in the refrigerator. The quinhydrone standards are highly unstable. They must be made fresh and discarded after use.
Light's Solution
39.21g ferrous ammonium sulphate, Fe(NH4)2(SO4)2·6H2O
48.22g ferric ammonium sulphate, Fe(NH4)(SO4)2·12H2O
56.2mL sulphuric acid (sp. gr. 1.84)
made to 1L with distilled water
Table 7: Light’s Solution (Ref: Ag/AgCl Saturated KCl)
|
Temperature (ºC) |
Potential (mV) |
|---|---|
|
25 |
476 |
ZoBell's Solution
1.408g potassium ferrocyanide, K4Fe(CN)6·3H2O
1.098g potassium ferricyanide, K3Fe(CN)6
7.456g potassium chloride, KCl
made to 1L with distilled water
Note:ZoBell’s solution is more stable prepared in two parts, which are accurately combined 1:1 just prior to use.
Part 1: 1.408g potassium ferrocyanide plus 7.456g of potassium chloride made up to 500mL.
Part 2: 1.098g potassium ferricyanide made up to 500mL.
Table 8:ZoBell’sSolution vs Ag/AgCl/Sat KCl Reference)
|
Temperature (ºC) |
Potential (mV) |
|
5 |
273 |
|
10 |
262 |
|
15 |
251 |
|
20 |
240 |
|
25 |
229 |
|
30 |
218 |
pH 4 Quinhydrone Solution
Add sufficient quinhydrone to NBS pH 4.01 buffer to make a saturated solution.
pH 6.86 Quinhydrone Solution
Add sufficient quinhydrone to NBS pH 6.87 buffer to make a saturated solution.
pH 9.18 Quinhydrone Solution
Add sufficient quinhydrone to NBS pH 9.18 buffer to make a saturated solution.
Table 9:Quinhydrone Solution (Ref: Ag/AgCl/ Saturated KCl)
|
Temperature (ºC) |
Potential (mV) |
||
|
pH 4.01 |
pH 6.87 |
pH 9.18 |
|
|
20 |
+268 |
+105 |
-23 |
|
25 |
+264 |
+98 |
-32 |
|
30 |
+260 |
+91 |
-41 |
